Service development and innovation (nuclear medicine physics)
In line with the Trust value of “Always Improving”, we strive to offer expertise to our clients by keeping up with the latest scientific research in all things nuclear medicine, reviewing clinical guidelines and literature. We are involved in local and multi-centre clinical trials, retrospective and prospective clinical audits and perform optimisation work involving the use of phantoms (brain phantoms, torso phantoms, Jaszczak phantoms and printed phantoms) and software (commercial as well as in-house).
Here's a taste of some of the recent projects we have worked on.
SIRT dosimetry
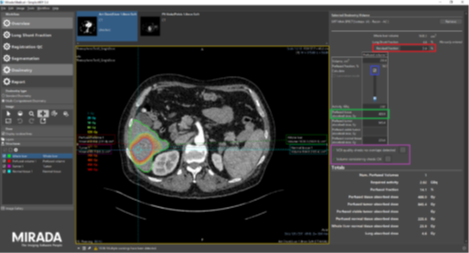
Selective internal radionuclide therapy (SIRT) delivers Y-90 microspheres intraarterially to treat liver tumours. Since 2022 physics perform pre-therapy dosimetry for SIRT using the Simplicity software (image below) to optimise individual patient treatment. Through extensive validation and cross-training we improved consistency and operator agreement. Working closely with the interventional radiologists and nuclear medicine consultants, we managed to escalate the treatment dose in line with recent guidelines through selective targeting which has resulted in reduced tumour burden and improved patient outcomes.
Improving scan time with “acquire during step” mode
In the past three years we worked on validating a new mode of SPECT/CT scanning called ‘acquire during step’ (ADS). After hours and hours of testing with Tc-99m-filled phantoms, and an award-winning presentation at the British Nuclear Medicine Society (BNMS) Spring meeting, we have now implemented ADS mode in the majority of our diagnostic SPECT/CTs, saving a minimum of 12.5% on scan time in each case. Following work with Tc-99m, we have also now validated its use for Lu-177 scanning, vastly improving scan time for our PRRT and PSMA therapy patients.
PSMA past and future at UHS
UHS became one of the first centres in the UK to provide this treatment as part of the phase III VISION trial which showed that PSMA improves quality of life and overall survival. This trial led to the licensing of Lu-177 PSMA by the MHRA and we are currently offering this treatment to a limited number of patients with the hope that it will be available for wider use in the future.
In the meantime, we have worked to optimise our procedures by:
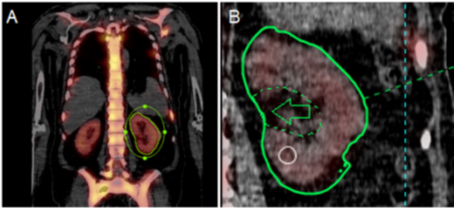
- Implementing a single-time-point dosimetry method, based on published evidence and local protocol development. This allows us to evaluate toxicity of PSMA treatment to the kidneys and salivary glands, and effectiveness of treatment by evaluating tumour doses (see image below).
- Improving quantitative accuracy of dosimetry. We calibrated the scanners in nuclear medicine to accurately quantify activity and established factors to account for partial volume effects which improve accuracy for small regions (like the salivary glands). The results of this work have been incorporated in our PSMA dosimetry process and were also presented at the BNMS Spring meeting in 2023.
- Optimising SPECT/CT imaging to reduce scan time whilst maintaining quantitative accuracy for dosimetry. Post-therapy Lu-177 PSMA imaging requires a SPECT/CT scan from eyes-to-thighs to assess where the radioactivity has accumulated and to track how the disease has changed from cycle to cycle. Through protocol optimisation and phantom validation, we were able to maintain the quantitative accuracy of scans, whilst reducing scan time from 80 to 33 minutes. This optimisation work has enabled us to improve patient comfort and create additional capacity for PSMA imaging. We presented our results to the BNMS Spring meeting in 2023.